Abstract
Background
Solitary bone plasmacytoma (SBP) is a rare form of plasma cell (PC) dyscrasia characterised by a single localised intraosseous mass of monoclonal PCs. Localised fractionated radical radiotherapy (RT) has remained standard of care, although a high proportion of patients progress to symptomatic myeloma. We and others have previously shown that occult marrow disease is demonstrable with multiparameter flow cytometry (FC) in a majority of patients and that this along with an abnormal serum free light chain (SFLC) ratio is associated with risk of progression and may be used to define a high-risk cohort.
Aim
IDRIS evaluated whether adjuvant immunomodulatory therapy with lenalidomide and dexamethasone (len+dex) after RT can improve progression free survival (PFS) in patients with high risk SBP compared with RT only.
Method
IDRIS is a randomised, open label, multicenter phase III study (trial ID; NCT02544308). Newly diagnosed, histologically confirmed SBP patients were enrolled. All patients were screened with cross-sectional imaging prior to study entry and all received standardised RT (45Gy in 1.8Gy fractions). High risk patients were randomised 1:1 to receive adjuvant therapy with len+dex or no further treatment. Adjuvant treatment consisted of 9 cycles of lenalidomide 25mg (day 1-21 of a 28 day cycle) and dexamethasone 20mg (weekly). The primary end point was PFS and secondary end points included overall survival, normalisation of bone marrow disease and SFLC ratio, time to next treatment, safety and toxicity. Recruitment was planned for 140 patients over 4 years at 35 sites, with the aim to randomise 98 high risk patients. The trial closed early due to low recruitment rate and the impact of the COVID 19 pandemic with a total of 36 patients registered at 12 sites.
Results
36 SBP patients were registered and 28 patients were identified as high risk. 26 high risk patients were randomised to receive adjuvant treatment with len+dex (n=13) or no further treatment (n=13). At baseline 77% in the treatment arm and 69% in the control arm demonstrated an aberrant PC phenotype by FC with medians of 0.11% (range 0.02-2.34%) and 0.29% (range 0.03-5.04). Abnormal SFLC ratio was detected in 62% of the treatment arm and 85% in the control arm with medians of 2.02 (range 0.12-5.16) and 2.64 (range 0.13-25).
12/13 patients started len+dex treatment with median duration of treatment being 252 days (range 84-266). 75% of patients who commenced treatment completed 9 cycles of lenalidomide and 67% completed 9 cycles of dexamethasone. All patients experienced grade 1/2 adverse events, the most common being fatigue, constipation, upper respiratory tract infections and insomnia. 3 patients experienced grade 3 adverse events; diarrhoea, thrombosis, raised gamma glutamyl transferase and malignancy. 2 patients discontinued treatment at cycle 5 due to disease progression and splenic thrombosis. 1 patient developed chronic myeloid leukaemia but this was not considered to be secondary to lenalidomide.
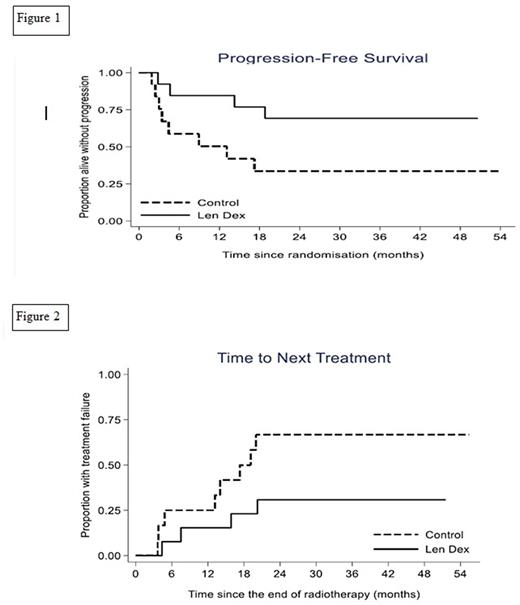
There was some evidence the treatment arm demonstrated a prolonged PFS compared with the control arm despite the lower than planned sample size (median not reached v 13 months; hazard ratio (HR) 0.32; 95% confidence interval (CI) 0.09-1.07; p=0.06) see Figure 1. In the control arm 8 patients progressed; 7 developed myeloma and 1 SBP within the RT field. In the treatment arm 4 patients progressed; 3 to myeloma and 1 to extramedullary plasmacytoma outside the RT field. Median time to next treatment was not reached in the treatment arm vs 17.3 months in the control arm (HR 0.34;95% CI 0.10-1.15; p = 0.08) see Figure 2. Overall survival was not calculated as there were no deaths. Following treatment with len+dex, 2/10 patients with aberrant PC phenotype at baseline had no detectable disease (sensitivity <0.001%) and the median PC% in those with persistent disease was 0.16% (range 0.007-0.33). Following len+dex 83% (95% CI: 52% to 98%) normalised the SFLC ratio. 55% (95% CI: 23% to 83%) of patients who had a detectable serum paraprotein at baseline achieved an immunofixation negative response post len+dex treatment.
Conclusion
These results indicate the potential beneficial effect of adjuvant systemic therapy following RT in patients with high risk SBP. This PFS benefit was demonstrable despite the limited numbers of patients included and relatively low intensity of systemic therapy received. Further studies are warranted in this area.
Disclosures
D'Sa:Janssen; Sanofi; BeiGene: Consultancy; janssen; beigene: Other: grant funding . Jackson:BMS: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; J and |J: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria; GSK: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Oncopeptides: Consultancy. Gallop-Evans:Kyowa-Kirin: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Educational Support. Parbutt:Janssen: Honoraria; AstraZeneca: Honoraria. Owen:Astra-Zeneca: Honoraria; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal